General information
Champion™ Competent Cells are chemically competent cells, which were prepared by SMOBIO to make E. coli perform excellent transformation efficiency. Standard transformation protocol is recommended for large plasmids or non-ampicillin selection. Time-saving transformation protocol is recommended for simple and rapid transformation. Champion™ Competent Cells are one of the fastest and simplest ready-to-use competent cell products in the world.
Kit contents
Champion™ Competent Cells
pUC19 Control Plasmid (5 μl, 10-4 μg/μl)
Champion™ Transformation Protocol Card
Shipping condition
Throughout the shipping process, the temperature is maintained under -70°C.
Storage and expiration
Champion™ Competent Cells must be stored between -70°C to -80°C. Subsequent freeze-thaw cycles will reduce transformation efficiency. If high efficiency is required for the experiment, do not use aliquots that have gone through several freeze-thaw cycles. The efficiency of Champion™ Competent Cells lasts for 1 year with proper storage.
Genotypes and applications
Product Name | Genotype | Application |
Champion™ 109 High | e14(McrA-)recA1 endA1 gyrA96 hsdR17(rk-, mK+) phoA supE44 thi-1 relA1 ∆(lac-proAB) (F’traD36 proAB lacIqZ∆M15 | Appropriate for blue-white color and robotic screening. It is a fast growing strain forming visible colonies within 8~10 hours. |
Champion™ 21 | F’ ompT hsdSβ(rβ-mβ-) dcm gal λ (DE3) | Appropriate host for recombinant protein expression using T7-based expression vectors. |
Champion™ DH5α High | recA1 endA1 gyrA96 hsdR17(rk-, mK+) phoA supE44 relA1 thi-1 ∆(lacZYA-argF)U 169 φ80 ∆(lacZ)M15 F- | Suitable for cloning with large plasmid and cDNA library construction, and also for blue-white colony selection. |
Items and ordering information
Product Name | Compatible to E. coli strain | Efficiency (cfu/μg) | Quantity | Cat. No. |
Champion™ 109 High | E. coli JM109 | >1 x 108 | 100 μl x 80 vials | CC0202 |
100 μl x 24 vials | CC0204 | |||
Champion™ 21 | E. coli BL21 (DE3) | >1 x 107 | 100 μl x 80 vials | CC2102 |
100 μl x 24 vials | CC2104 | |||
Champion™ DH5α High | E. coli DH5α | >3 x 108 | 100 μl x 80 vials | CC5202 |
100 μl x 24 vials | CC5204 |
Will transformation efficiency be reduced when Champion™ competent cells are thawed, dispensed and refrozen repeatedly?
When Champion™ competent cells are thawed dispensed in aliquots and refrozen within 1 min, the transformation efficiency will be 10~20% reduced compared to the first time use.
What is the advantage of thawing competent cells with circulating water instead of still water?
Using circulating water can reduce the thawing time. Less thawing time shows higher efficiency than long thawing time.
Is there any difference of transformation efficiency between using plating beads, bending glass rod and plating loop?
The bending glass rod shows best efficiency and the plating loop show less efficiency than other method. For handle a lot samples at the same time the plating beads are recommended.
Is 1 second vortex critical for mixture the DNA?
One second vortex provides more reliable transformation efficiency (1.1 times compare with mixed by finger tapping)
Will heat shock affect the transformation efficiency?
Heat shock treatment will enhance the efficiency about 1~2X versus non-heat shock method.

Factors Affecting Transformation Efficiency
Thawing methods
Shorter thawing time is more efficient than a longer thawing time. Slow thawing caused by power shortages and unstable freezers will result in decreased efficiency.
Size of plasmid
Plasmid size affect the efficiency greatly. The efficiency of transforming a supercoiled 2.7 kb is approximately 100 times higher than that of a 10 Kb plasmid (using time-saving transformation protocol). For large plasmids (> 6 kb), standard transformation protocol is recommended.
Heat shock treatment
Heat shock treatment will enhance the efficiency about 1~2 folds versus non-heat shock method.
Plating methods
Bent glass rods show the greatest efficiency, while plating loop shows less efficiency than plating beads. When handling a large quantity of samples at the same time, plating beads are recommended.
Concentration of antibiotic
Antibiotic concentration is critical to use of the time-saving transformation protocol.
Antibiotic | Concentration |
Ampicillin (Ap) | 20 μg/ml |
Kanamycin (Km) | 25 μg/ml |
Tetracycline (Tc) | 7.5 μg/ml |
Chloramphenicol (Cm) | 20 μg/ml |
For plasmid size <6 Kb, the efficiency of kanamycin selection is usually 3~10 times less than the ampicillin selection. For plasmid size > 6 Kb, the efficiency of kanamycin selection is much lower than ampicillin. We suggest using the standard transformation protocol (with heat shock and recovery steps) to enhance the efficiency.
Product Name | Compatible to E. coli strain | Efficiency (cfu/μg) | Quantity | Cat. No. |
Champion™ 109 High | E. coli JM109 | >1 x 108 | 100 μl x 80 vials | |
100 μl x 24 vials | ||||
Champion™ 21 | E. coli BL21 (DE3) | >1 x 107 | 100 μl x 80 vials | |
100 μl x 24 vials | ||||
Champion™ DH5α High | E. coli DH5α | >3 x 108 | 100 μl x 80 vials | |
100 μl x 24 vials |
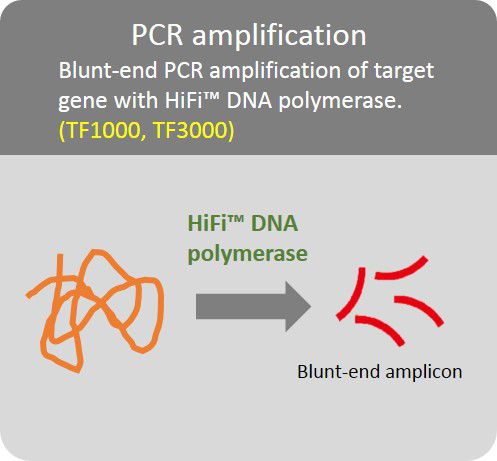
High Fidelity PCR amplification
Amplification of target gene with HiFi™ DNA polymerase to minimize error rate.

Gel electrophoresis
Staining amplicons with safe fluorescent dyes, following by observation under blue-light illuminator to minimize damage of DNA amplicons and maximize successful cloning efficiency.
Safe fluorescent dyes
[NS1000] FluoroVue™ Nucleic Acid Gel Stain (10,000X), 500 μl
[DS1000] FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X), 500 μl
[DL5000] FluoroDye™ DNA Fluorescent Loading Dye (Green, 6X), 1 ml
Blue-light illuminator

Ligation
Blund-end PCR amplicons can directly ligate with PCR cloning vector.
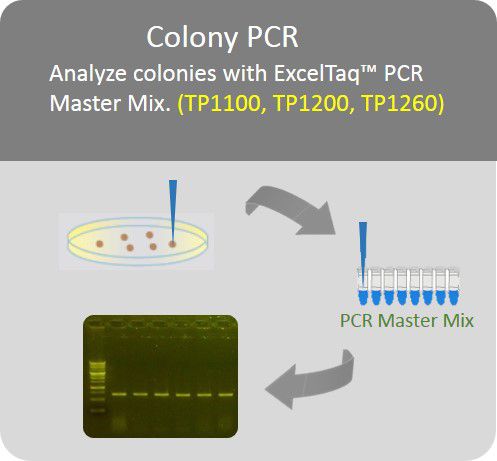
Colony PCR
Analyze colonies with PCR master mix to save preparation time.

![[CC5204] Champion™高效能DH5α勝任細胞, 24管](/web/image/product.template/415/image?unique=0561fe8)


